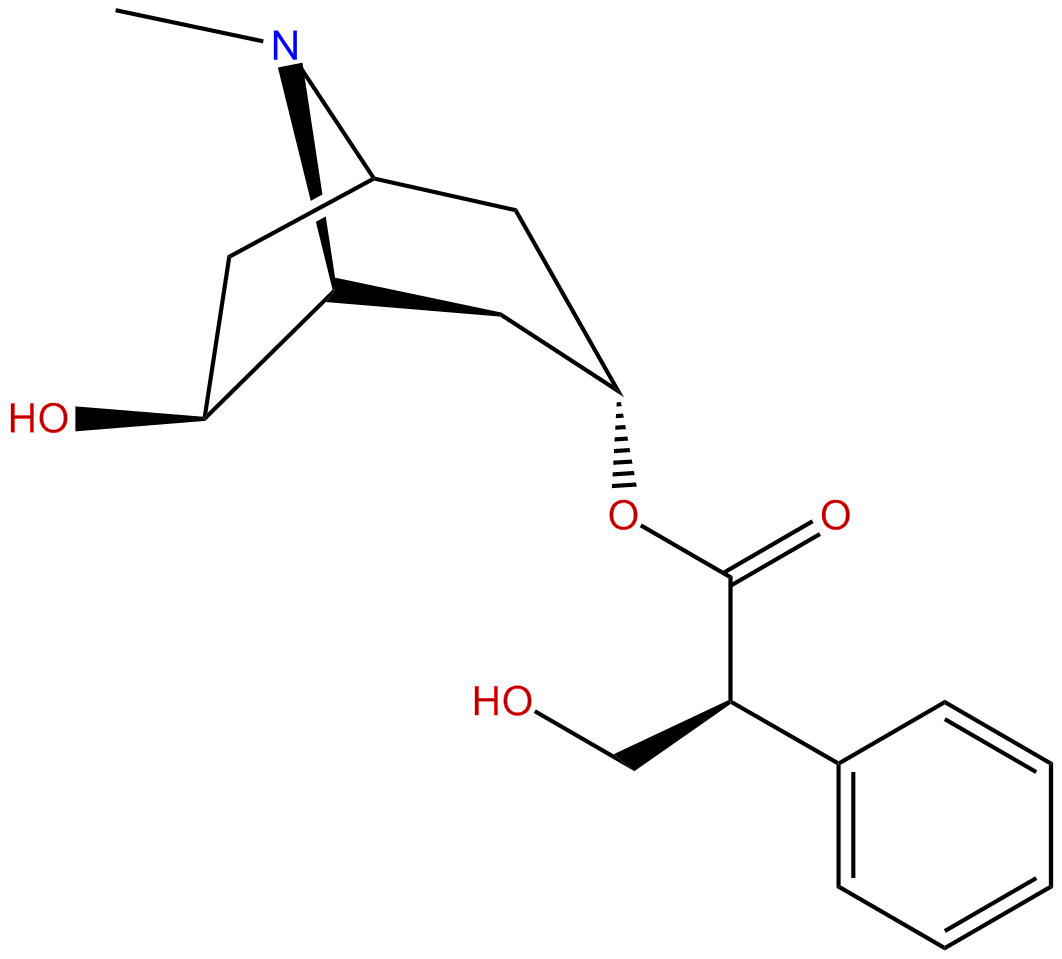
anisodamineCAS No.:17659-49-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP0174 |
| Formula: | C17H23NO4 |
| Mol Weight: | 305.36882 |
Synonym name: Raceanisodamine; 6-Hydroxyhyoscyamine; 6-beta-Hydroxyhyoscyamine
Catalogue No.: BP0174
Cas No.: 17659-49-3
Formula: C17H23NO4
Mol Weight: 305.374
Botanical Source:
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Can be supplied from milligrams to grams.
For Reference Standard and R&D, Not for Human Use Directly.
Inquire for bulk scale.
Description:
Anisodamine, an anticholinergic drug, has antishock effect, which is intimately linked to alpha7nAChR-dependent anti-inflammatory pathway. Anisodamine demonstrates a direct cardiac depressive action at the myocyte level, which may be related to, at least in part, NO production and cholinoceptor antagonism, it causes the changes of structure and function in the transmembrane domain of the Ca(2+)-ATPase from sarcoplasmic reticulum. Anisodamine, a vasoactive drug, can abate endogenous endotoxaemia subsequent to splanchnic vasoconstriction due to hypovolaemia, it alleviates inflammatory damage by significantly reducing the expressions of VEGF and ICAM-1, and shows significant protective effects in an animal model of infusion phlebitis. Anisodamine also inhibits shiga toxin type 2-mediated tumor necrosis factor-alpha production in vitro and in vivo.
References:
Burns. 1997 Mar;23(2):142-6.
Anisodamine restores bowel circulation in burn shock.
METHODS AND RESULTS:
In a group of eight burn patients with a mean of 65.3 +/- 17.4 per cent TBSA burn injury (range 50-90 per cent TBSA), accompanied by a mean of 43.5 +/- 18.9 per cent TBSA full-thickness injury, it was shown that the evidence of global hypovolaemia had disappeared at 12 h after the injury following aggressive fluid resuscitation, while there was still a subnormal pHi of stomach at 48 h. As a prolonged period of inadequacy of oxygen delivery to the intestine might result in impairment of the intestinal mucosal barrier function, and then endogenous endotoxaemia might ensue, it seems to be important to correct intestinal hypoxia as early as possible. Since the inadequate perfusion to the gut wall is due to selective vasoconstriction of the mesenteric vasculature, logic dictates that the use of a vasodilator is in order. Anisodamine, an anticholinergic drug, was then given in six burn patients with comparable burn size and amount of fluid replenishment with the eight patients in the control group. It was clearly demonstrated that gastric pHi returned to normal before 48 h after injury. Plasma endotoxin and TNF contents were measured, and they were significantly lower than control values after 72 h.
CONCLUSIONS:
In conclusion, it is believed that Anisodamine might be a valuable adjunct to the resuscitation regime of burn shock, and, therefore, a promising drug to abate endogenous endotoxaemia subsequent to splanchnic vasoconstriction due to hypovolaemia. The shortcomings of the drug were a mild abdominal distention and tachycardia after its administration.
Exp Biol Med (Maywood). 2001 Jun;226(6):597-604.
Anisodamine inhibits shiga toxin type 2-mediated tumor necrosis factor-alpha production in vitro and in vivo.
Cytokines, in particular tumor necrosis factor (TNF), appear to be necessary to develop the pathological process of Shiga toxin-producing Escherichia coli (STEC) infection.
METHODS AND RESULTS:
In this study we examined the effect of Anisodamine, a vasoactive drug, on TNF-alpha production in Shiga toxin type 2 (Stx2)-stimulated human monocytic cells in vitro and in Stx2-injected mice sera in vivo. Human monocytes and THP-1 cells were stimulated by Stx2 (1-100 ng/ml) with or without Anisodamine addition (1-400 micrograms/ml). For in vivo evaluations, C57BL/6 mice were given a single intraperitoneal injection of Anisodamine (6-50 mg/kg) or saline after intraperitoneal injection of Stx2 (50 ng/kg). The results showed that Anisodamine suppressed Stx2-induced TNF-alpha production in a dose- and time-dependent manner. Anisodamine also suppressed Stx2-induced TNF-alpha mRNA expression. Further study showed that endogenous prostaglandin E2 may be involved in this inhibitory effect. In contrast to TNF-alpha mRNA, Anisodamine at concentrations as high as 400 micrograms/ml did not decrease Stx2-induced IL-1 beta and IL-8 mRNA levels. In addition, Anisodamine (> 50 micrograms/ml) increased Stx2-stimulated THP-1 cell viability. Levels of TNF-alpha in Anisodamine-treated mice sera were significantly lower than those in the saline-treated group 1.5 and 24 hr after Stx2 injection. Anisodamine induced a lower percentage of death in Stx2-injected mice.
CONCLUSIONS:
Taken together, our results indicate that Anisodamine has an important regulatory effect on Stx2-induced TNF-alpha production in vitro and in vivo. The present study suggested that this drug should be further investigated for its effects on Stx2-mediated diseases in humans.
Chin Med J (Engl). 2012 Jan;125(2):300-5.
Effects of anisodamine on the expressions of vascular endothelial growth factor and intercellular adhesion molecule 1 in experimental infusion phlebitis.
Infusion phlebitis is the most common side effect of clinical intravenous drug therapy and several clinical studies have demonstrated that Anisodamine can effectively prevent the occurrence of infusion phlebitis. This study was designed to investigate effects of Anisodamine on the expressions of vascular endothelial growth factor (VEGF) and intercellular adhesion molecule 1 (ICAM-1) in a rabbit model of infusion phlebitis and to analyze the mechanisms of Anisodamine effect on the prevention and treatment of experimental infusion phlebitis.
METHODS AND RESULTS:
Twenty-four specific pathogen-free male Japanese white rabbits were randomly assigned to the control group, the model group, the magnesium sulfate group and the Anisodamine group. The rabbit model of infusion phlebitis, induced by intravenous administration, was established and expressions of VEGF and ICAM-1 were determined and contrasted with the control group treated with normal saline. We evaluated expression by histopathology, immunohistochemistry, reverse transcription-polymerase chain reaction, and Western blotting assay.Pathohistological changes of the model group were observed, such as loss of venous endothelial cells, inflammatory cell infiltration, edema and thrombus. The magnesium sulfate group and the Anisodamine group showed significant protective effects on vascular congestion, inflammatory cell infiltration, proliferation, swelling of endothelium and perivascular hemorrhage. The model group showed the highest expressions of VEGF and ICAM-1 of the four groups (P < 0.01). On the contrary, Anisodamine alleviated the inflammatory damage by significantly reducing the expressions of VEGF and ICAM-1 compared with the model group (P < 0.01). There was no significant difference in the expressions of VEGF and ICAM-1 between the magnesium sulfate group and the Anisodamine group (P > 0.05).
CONCLUSIONS:
Anisodamine alleviates inflammatory damage by significantly reducing the expressions of VEGF and ICAM-1, and shows significant protective effects in an animal model of infusion phlebitis.
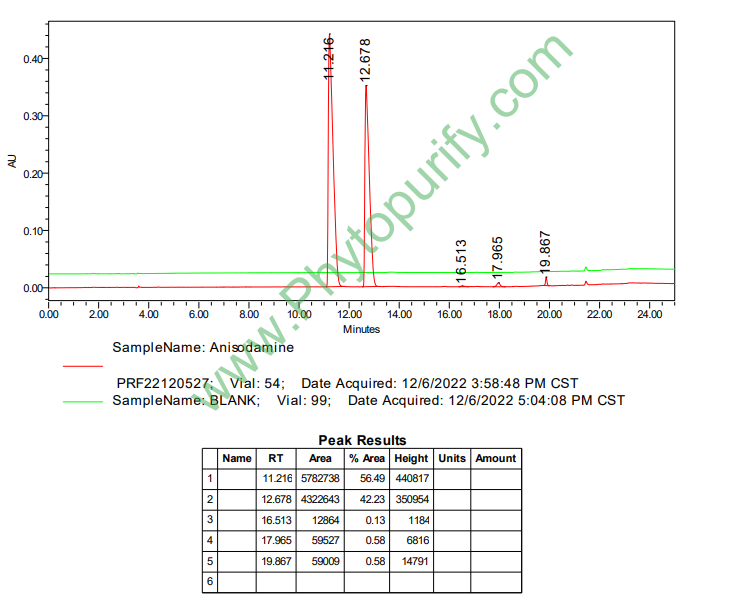
HPLC of Anisodamine