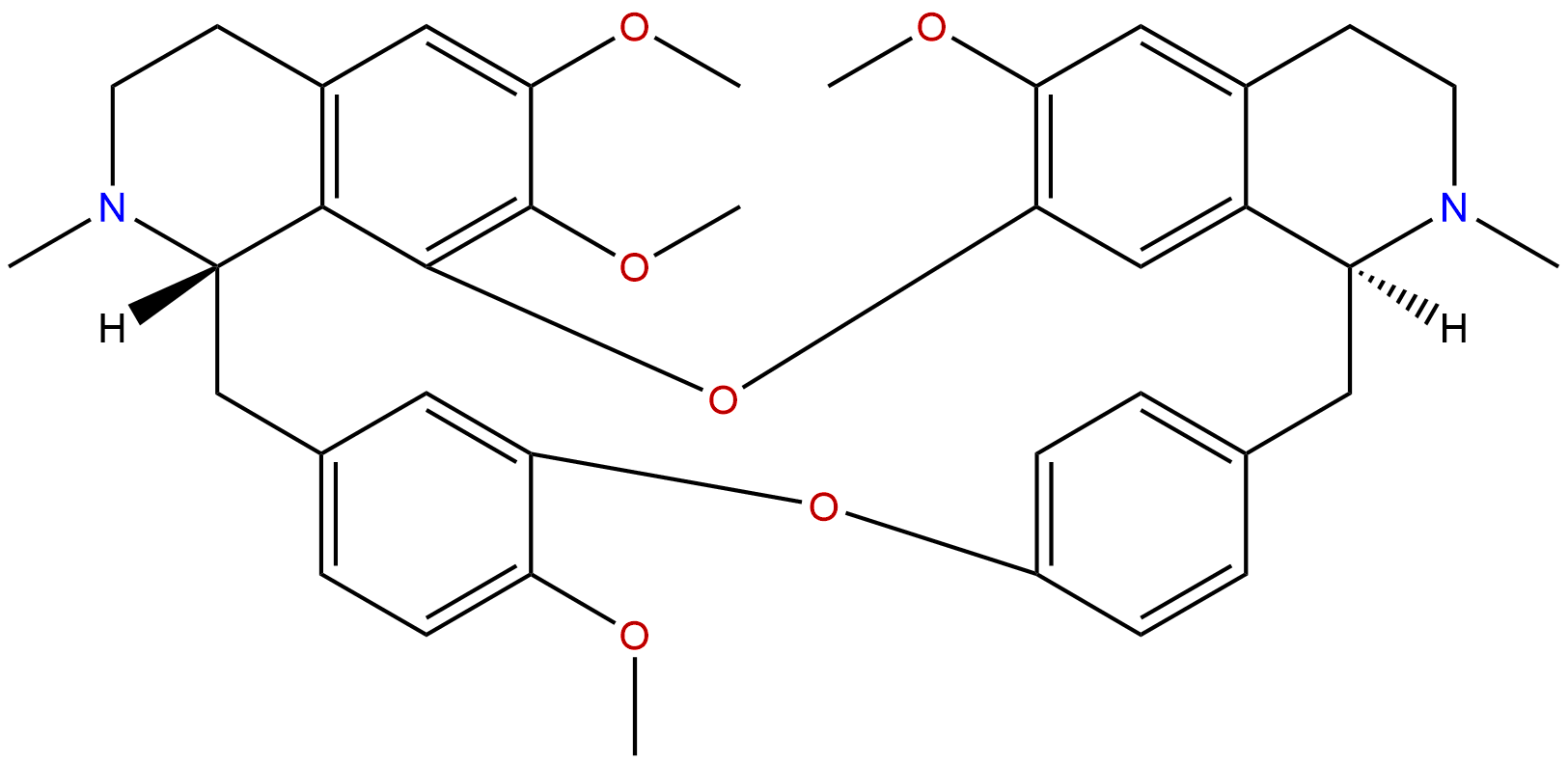
TetrandrineCAS No.:518-34-3
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1379 |
| Formula: | C38H42N2O6 |
| Mol Weight: | 622.762 |
Product name: Tetrandrine
Synonym name:
Catalogue No.: BP1379
Cas No.: 518-34-3
Formula: C38H42N2O6
Mol Weight: 622.762
Botanical Source: Stephaniae Tetrandrae Radix
Physical Description:
Type of Compound: Alkaloids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Tetrandrine is a calcium channel blocker, which shows antitumor, antifibrotic, anti-oxidant, anti-inflammatory and immunosuppressive activity. It suppressed Wnt/β-catenin signaling transduction, the migration of DU145 and PC-3 cells, EOMA cell growth through the ROS/Akt pathway and inhibited inward rectifying potassium current in cultured bovine aortic endothelial cells.
References:
Asian J Androl. 2015 Feb 6.
Tetrandrine suppresses proliferation, induces apoptosis, and inhibits migration and invasion in human prostate cancer cells.
Tetrandrine (TET), a traditional Chinese medicine, exerts remarkable anticancer activity on various cancer cells. However, little is known about the effect of TET on human prostate cancer cells, and the mechanism of function of TET on prostate cancer has not yet been elucidated.
METHODS AND RESULTS:
To investigate the effects of TET on the suppression of proliferation, induction of apoptosis, and inhibition of migration and invasion in human prostate cancer cell lines, DU145 and PC-3. Inhibition of growth was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and clone formation assay, and flow cytometry analysis was performed to detect the induction of apoptosis. Activation of poly (ADP-ribose) polymerase, caspase-3, Akt, phospho-Akt, Bcl-2, and Bax was analyzed by Western blotting. Wound healing assay and transwell migration assay were used to evaluate the effect of TET on migration and invasion of cancer cells. TET inhibited the growth of DU145 and PC-3 cells in a dose- and time-dependent manner. Cell cloning was inhibited in the presence of TET in DU145 and PC-3 cells. TET suppressed the migration of DU145 and PC-3 cells. Transwell invasion assay showed that TET significantly weakened invasion capacity of DU145 and PC-3 cells. TET exhibited strong inhibitory effect on proliferation, migration, and invasion of prostate cancer cells. In addition, TET induced apoptosis in a dose-dependent manner by activating the caspase cascade and inhibiting phosphoinositide 3-kinase-Akt signal pathway.
CONCLUSIONS:
The accumulating evidence suggests that TET could be a potential therapeutic candidate against prostate cancer in a clinical setting.
Acta Pharmacol Sin. 2000 Dec;21(12):1115-8.
Tetrandrine inhibits inward rectifying potassium current in cultured bovine aortic endothelial cells.
To study the effect of Tetrandrine (Tet) on inward rectifying potassium current in cultured bovine aortic endothelial cells.
METHODS AND RESULTS:
Inward rectifying potassium current (IRK) was observed by the whole cell patch-clamp technique. IRK was inhibited by Tet in a concentration-dependent manner and recovered to normal after wash with drug-free external solution. IRK was reduced from (582 +/- 48) pA to (221 +/- 40) pA at a holding potential of -70 mV by Tet 30 mumol/L. IC50 was 2.8 mumol/L.
CONCLUSIONS:
Tet inhibited inward rectifying potassium current in cultured bovine aortic endothelial cells.
J Gastroenterol Hepatol. 2007 Jan;22(1):99-111.Antifibrotic effects of tetrandrine on hepatic stellate cells and rats with liver fibrosis.
Tetrandrine (TET), a traditional Chinese medicine, exerts remarkable anticancer activity on various cancer cells. However, little is known about the effect of TET on human prostate cancer cells, and the mechanism of function of TET on prostate cancer has not yet been elucidated.
METHODS AND RESULTS:
Anti-inflammation strategies are one of the proposed therapeutic approaches to hepatic fibrosis. Tetrandrine (C(38)H(42)O(8)N(2), molecular weight: 622; Tet), an alkaloid isolated from the Chinese medicinal herb Stephania tetrandra, has been shown to exert anti-inflammatory activity in pulmonary diseases. The purpose of the present study was to investigate the in vitro and in vivo effects of Tet on hepatic fibrosis.
METHODS AND RESULTS:
A cell line of rat hepatic stellate cells (HSC-T6) was stimulated with transforming growth factor-beta1 (TGF-beta1) or tumor necrosis factor-alpha (TNF-alpha). The inhibitory effects of Tet on the nuclear factor kappaB (NFkappaB) signaling cascade and molecular markers including intercellular adhesion molecule-1 (ICAM-1) and alpha-smooth muscle actin (alpha-SMA) secretion were assessed. Fibrosis was induced by dimethylnitrosamine (DMN) administration in rats for 4 weeks. Fibrotic rats were randomly assigned to one of the four groups: vehicle (0.7% carboxyl methyl cellulose, CMC), Tet (1 mg/kg), Tet (5 mg/kg), or silymarin (50 mg/kg), each given by gavage twice daily for 3 weeks starting after 1 week of DMN administration. At the end of the study, liver tissues were scored for fibrosis and analyzed for molecular markers of fibrosis. Tetrandrine (0.5-5.0 micromol/L) concentration-dependently inhibited NFkappaB transcriptional activity induced by TNF-alpha, including IkappaBalpha phosphorylation and mRNA expressions of ICAM-1 in HSC-T6 cells. In addition, Tet also inhibited TGF-beta1-induced alpha-SMA secretion and collagen deposition in HSC-T6 cells. Fibrosis scores of livers from DMN-treated rats with high-dose Tet (1.3 +/- 0.3) were significantly reduced in comparison with DMN-treated rats receiving saline (2.0 +/- 0.2). Hepatic collagen content of DMN rats was significantly reduced by either Tet or silymarin treatment.
CONCLUSIONS:
Double-staining results showed that alpha-SMA- and NFkappaB-positive cells were decreased in the fibrotic livers by Tet and silymarin treatment. In addition, mRNA expression of ICAM-1, alpha-SMA, and TGF-beta1 was attenuated by Tet treatment. Moreover, levels of plasma aspartate aminotransferase and alanine aminotransferase activities were reduced by Tet and silymarin treatment.
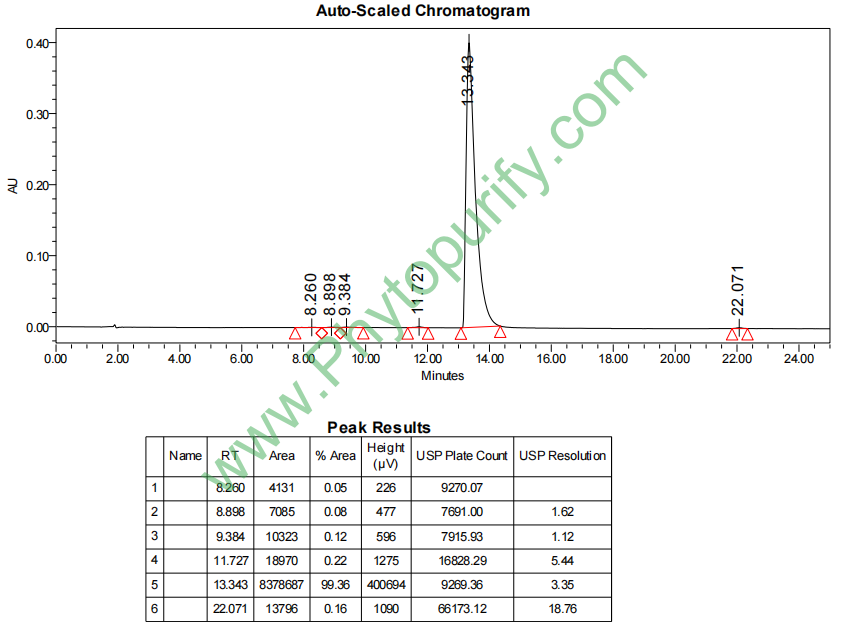
HPLC of Tetrandrine