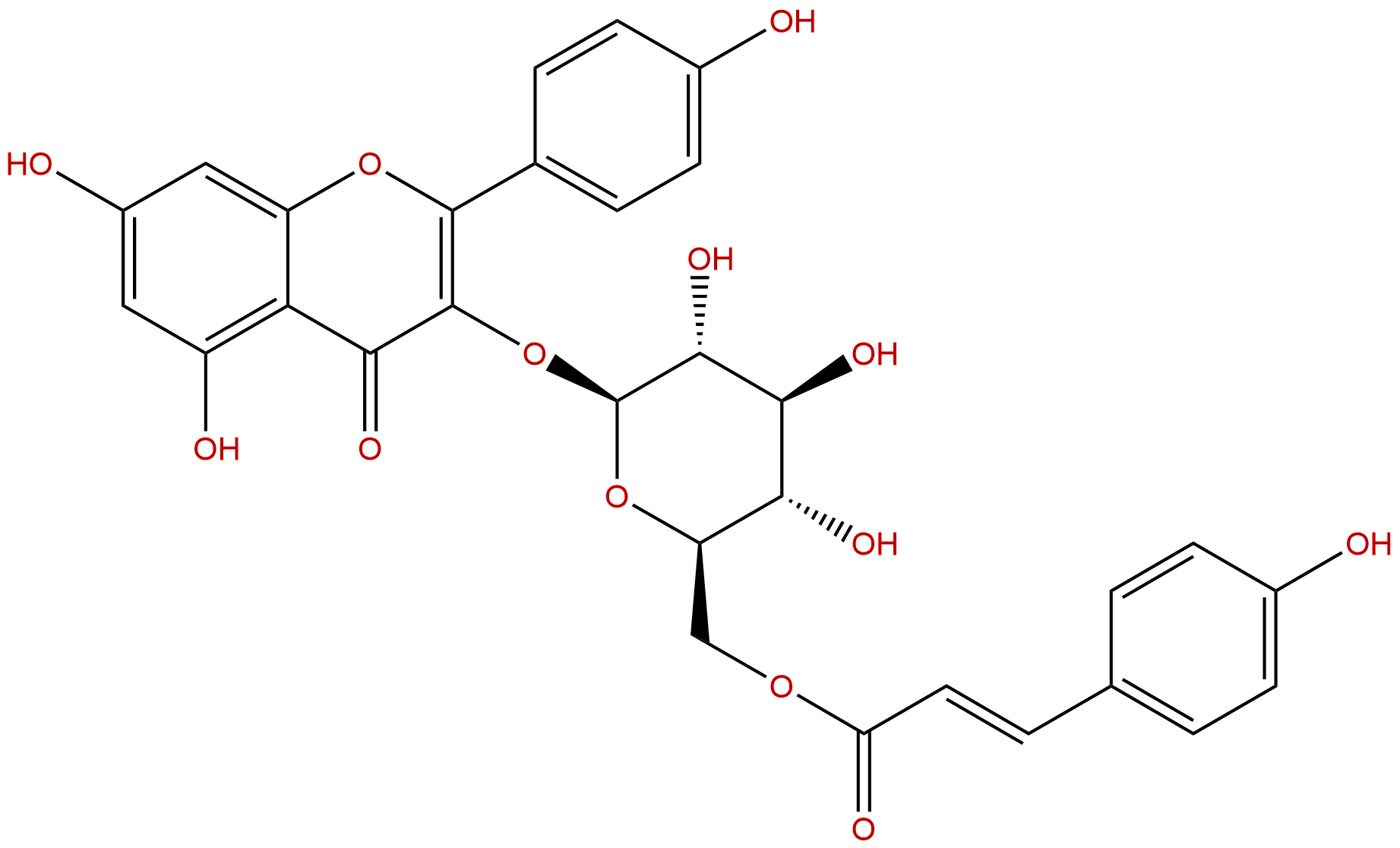
TilirosideCAS No.:20316-62-5
|
||||||||||
 |
|
|
||||||||

| Catalogue No.: | BP1393 |
| Formula: | C30H26O13 |
| Mol Weight: | 594.525 |
Product name: Tiliroside
Synonym name: 6′′-O-trans-p-Coumaroylastragalin
Catalogue No.: BP1393
Cas No.: 20316-62-5
Formula: C30H26O13
Mol Weight: 594.525
Botanical Source: Edgeworthia chrysantha Lindl.
Physical Description:
Type of Compound: Flavonoids
Purity: 95%~99%
Analysis Method: HPLC-DAD or/and HPLC-ELSD
Identification Method: Mass, NMR
Packing: Brown vial or HDPE plastic bottle
Storage: Store in a well closed container, protected from air and light. Put into refrigerate or freeze for long term storage.
Whenever possible, you should prepare and use solutions on the same day. However, if you need to make up stock solutions in advance, we recommend that you store the solution as aliquots in tightly sealed vials at -20℃. Generally, these will be useable for up to two weeks.
The product could be supplied from milligrams to grams
Inquire for bulk scale.
Description:
Tiliroside possesses anti-inflammatory, antioxidant, anti-complement, anti-diabetic, anticarcinogenic and hepatoprotective activities. Tiliroside enhances fatty acid oxidation via the enhancement adiponectin signaling associated with the activation of both AMP-activated protein kinase and peroxisome proliferator-activated receptor α and ameliorates obesity-induced metabolic disorders.
References:
Fitoterapia. 2007 Jan;78(1):1-6.
Tiliroside and gnaphaliin inhibit human low density lipoprotein oxidation.
Two flavonoids, gnaphaliin and Tiliroside, isolated from Helichrysum italicum, were studied in vitro for their capacity to inhibit Cu(2+)-induced human low density lipoprotein (LDL) and diluted plasma oxidation.
METHODS AND RESULTS:
LDL oxidation was monitored by conjugated diene, thiobarbituric acid-reactive substances (TBARS) formation and electrophoretic mobility on agarose gel. Gnaphaliin and Tiliroside increased the lag-phase for diene conjugate production in a dose-dependent manner. The reduction of TBARS production confirmed the antioxidant activity of gnaphaliin and Tiliroside with 50% inhibitory concentration (IC(50)) values of 8.0+/-3.9 microM and 7.0+/-2.6 microM respectively. Furthermore, the flavonoids negated the Cu(2+)-induced increase in electrophoretic mobility of LDL. Antioxidant activity of gnaphaliin and Tiliroside was significantly different when diluted plasma was oxidised by adding 1 mM CuSO(4). Although both flavonoids again reduced the TBARS production, Tiliroside showed higher activity than gnaphaliin (IC(50)=10.6+/-2.5 microM vs. IC(50)>50 microM).
CONCLUSIONS:
In conclusion, Tiliroside and gnaphaliin are antioxidants against in vitro Cu(2+)-induced LDL oxidation in the same order of magnitude compared to that of the reference drug, probucol.
Eur J Pharmacol. 2003 Feb 7;461(1):53-61.
Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside.
Three flavonoids, gnaphaliin, pinocembrin and Tiliroside, isolated from Helichrysum italicum, were studied in vitro for their antioxidant and/or scavenger properties and in vivo in different models of inflammation.
METHODS AND RESULTS:
In vitro tests included lipid peroxidation in rat liver microsomes, superoxide radical generation in the xanthine/xanthine oxidase system and the reduction of the stable radical 1,1-diphenyl-2-pycryl-hydrazyl (DPPH). Acute inflammation was induced by application of 12-O-tetradecanoylphorbol 13-acetate (TPA) to the mouse ear or by subcutaneous injection of phospholipase A(2) or serotonin in the mouse paw. Eczema provoked on the mouse ear by repeated administration of TPA was selected as a model of chronic inflammation. The flavonoids were assayed against sheep red blood cell-induced mouse paw oedema as a model of delayed-type hypersensitivity reaction. The most active compound, both in vitro and in vivo, was Tiliroside. It significantly inhibited enzymatic and non-enzymatic lipid peroxidation (IC(50)=12.6 and 28 microM, respectively). It had scavenger properties (IC(50)=21.3 microM) and very potent antioxidant activity in the DPPH test (IC(50)=6 microM). In vivo, Tiliroside significantly inhibited the mouse paw oedema induced by phospholipase A(2)(ED(50)=35.6 mg/kg) and the mouse ear inflammation induced by TPA (ED(50)=357 microg/ear). Pinocembrin was the only flavonoid that exhibited anti-inflammatory activity in the sheep red blood cell-induced delayed-type hypersensitivity reaction. However, only Tilirosidesignificantly reduced the oedema and leukocyte infiltration induced by TPA.
CONCLUSIONS:
As in the case of other flavonoids, the anti-inflammatory activity of Tiliroside could be based on its antioxidant properties, although other mechanisms are probably involved.
Mol Nutr Food Res. 2012 Mar;56(3):435-45.
Tiliroside, a glycosidic flavonoid, inhibits carbohydrate digestion and glucose absorption in the gastrointestinal tract.
Recent studies have reported that Tiliroside, a glycosidic flavonoid, possesses anti-diabetic activities. In the present study, we investigated the effects of Tiliroside on carbohydrate digestion and absorption in the gastrointestinal tract.
METHODS AND RESULTS:
This study showed that Tiliroside inhibits pancreatic α-amylase (IC₅₀ = 0.28 mM) in vitro. Tiliroside was found as a noncompetitive inhibitor of α-amylase with K(i) values of 84.2 μM. In male ICR mice, the increase in postprandial plasma glucose levels was significantly suppressed in the Tiliroside-administered group. Tiliroside treatment also suppressed hyperinsulinemia after starch administration. Tiliroside administration inhibited the increase of plasma glucose levels in an oral glucose tolerance test, but not in an intraperitoneal glucose tolerance test. In human intestinal Caco-2 cells, the addition of Tiliroside caused a significant dose-dependent inhibition of glucose uptake. The inhibitory effects of both sodium-dependent glucose transporter 1 (SGLT1) and glucose transporter 2 (GLUT2) inhibitors (phlorizin and phloretin, respectively) on glucose uptake were significantly inhibited in the presence of Tiliroside, suggesting that Tiliroside inhibited glucose uptake mediated by both SGLT1 and GLUT2.
CONCLUSIONS:
These findings indicate that the anti-diabetic effects of Tiliroside are at least partially mediated through inhibitory effects on carbohydrate digestion and glucose uptake in the gastrointestinal tract.
Bioorg Med Chem. 2002 Mar;10(3):707-12.
Hepatoprotective principles from the flowers of Tilia argentea (linden): structure requirements of tiliroside and mechanisms of action.
The methanolic extract from the flowers of Tilia argentea (linden) was found to show a hepatoprotective effect against D-galactosamine (D-GalN)/lipopolysaccharide (LPS)-induced liver injury in mice.
METHODS AND RESULTS:
By bioassay-guided separation using in vitro D-GalN-induced damage to hepatocytes, five flavonol glycosides were isolated as the hepatoprotective constituents of the methanolic extract. Tiliroside, the principal flavonol glycoside, strongly inhibited serum GPT and GOT elevations at doses of 25-100 mg/kg (p.o.) in D-GalN/LPS-treated mice. By comparing the inhibitory effects of Tiliroside with those of its components alone, the kaempferol 3-O-beta-D-glucopyranoside moiety was found to be essential for the activity, and its effect was suggested to depend on the inhibition of tumor necrosis factor-alpha (TNF-alpha) production, decreased sensitivity of hepatocytes to TNF-alpha, and on the protection of hepatocytes against D-GalN.
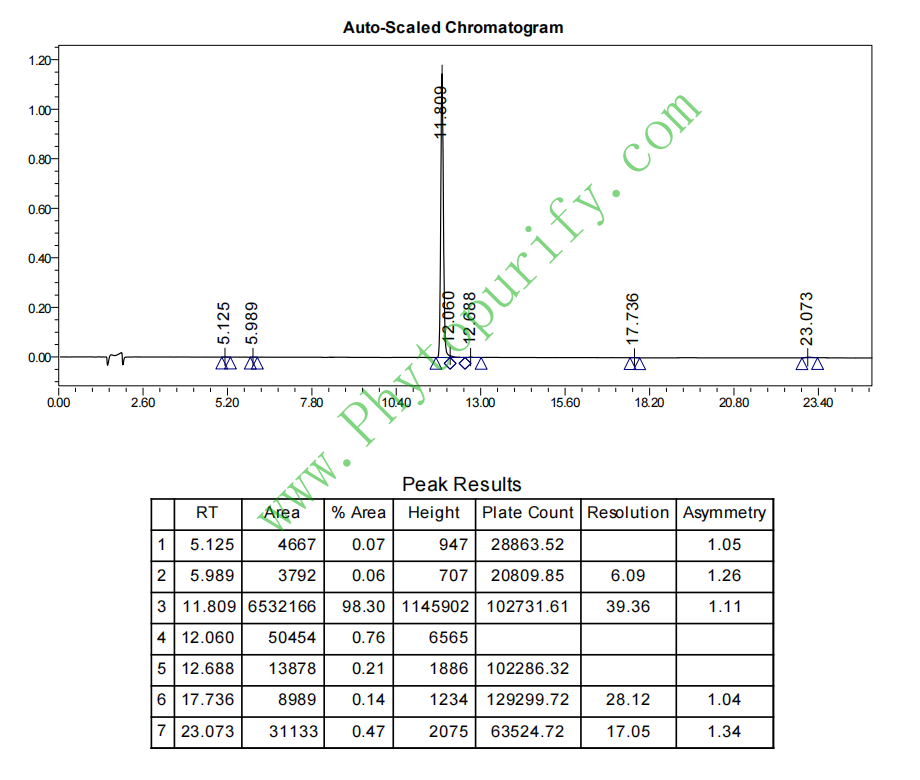
HPLC of Tiliroside